In the constantly evolving landscape of pharmaceutical manufacturing, efficiency, accuracy, and compliance are paramount. BatchLine Lite MES emerges as a pivotal tool to meet these demands. Let’s delve into how this innovative system can transform your manufacturing processes through streamlined functionalities and integrations.
General System Functionality
Revising Specific Parts of the eBMR
One of the standout features of BatchLine Lite MES is its flexibility in handling Electronic Batch Records (eBMR).
Users can revise specific sections, though the creation of a new revision encompasses the complete Master Batch Record (MBR).
This ensures a thorough review, approval, and activation process before it’s put to use.
BMR vs. BPR: Independent or Integrated?
Designing production batch records starts with crafting a Master Batch Record (MBR), serving as the guiding blueprint. The Batch Production Record (BPR) follows, documenting each execution phase.
Manufacturers have the freedom to develop BMR and BPR either independently or as a cohesive, integrated flow, tailored to operational strategies.
Differentiating Commercial and Pilot/Trial eBMRs
BatchLine Lite MES equips manufacturers to design MBRs for both commercial and non-commercial applications.
With customizable filtering, users can efficiently manage commercial eBRs distinct from pilot or trial batches, ensuring each is addressed with its unique requirements.
Sequential Approval of Manufacturing Steps
BatchLine Lite MES supports the configuration of manufacturing steps to be executed in sequence or parallel, complete with necessary reviews and verifications.
This configuration is precisely outlined during the MBR specification design workshop.
Integration Capabilities
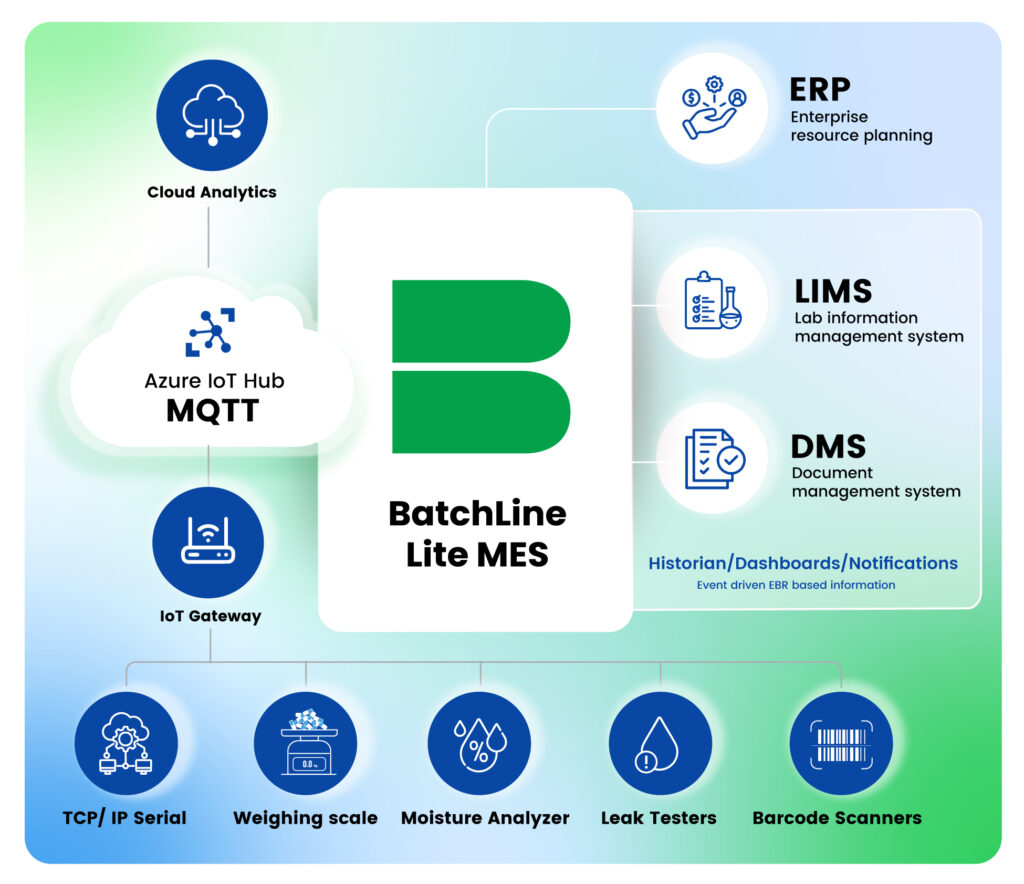
Integration with LIMS and SAP (ERP)
BatchLine Lite MES integrates seamlessly with Laboratory Information Management Systems (LIMS) and Enterprise Resource Planning (ERP) systems via REST API connectors. This fluid data exchange optimizes operations.
- LIMS Use Case: Enables automatic transfer of lab results and QC data into batch records, utilizing only tested and approved materials.
- ERP Use Case: Syncs production schedules and material consumption data, ensuring cohesive operations across platforms.
Integrating Weighing Scales and Barcode Scanners
The system supports integration with weighing scales, employing the MQTT protocol via a Gateway to interface effectively.
Additionally, it incorporates barcode scanners, enhancing data entry’s efficiency and accuracy.
Instrument Integration for Varied Tests
Incorporating data from cloud-based instruments like leak testers or moisture analyzers seamlessly into the eBMR ensures no manual entry is left unchecked, streamlining the review and approval process.
Operation and Continuity
Managing Deviations in Manufacturing
BatchLine Lite MES adeptly manages production exceptions, requiring reviews and approvals by supervisors and QA.
Outcomes like Corrective and Preventive Actions (CAPA) are addressed using the customer’s eQMS, post-investigation.
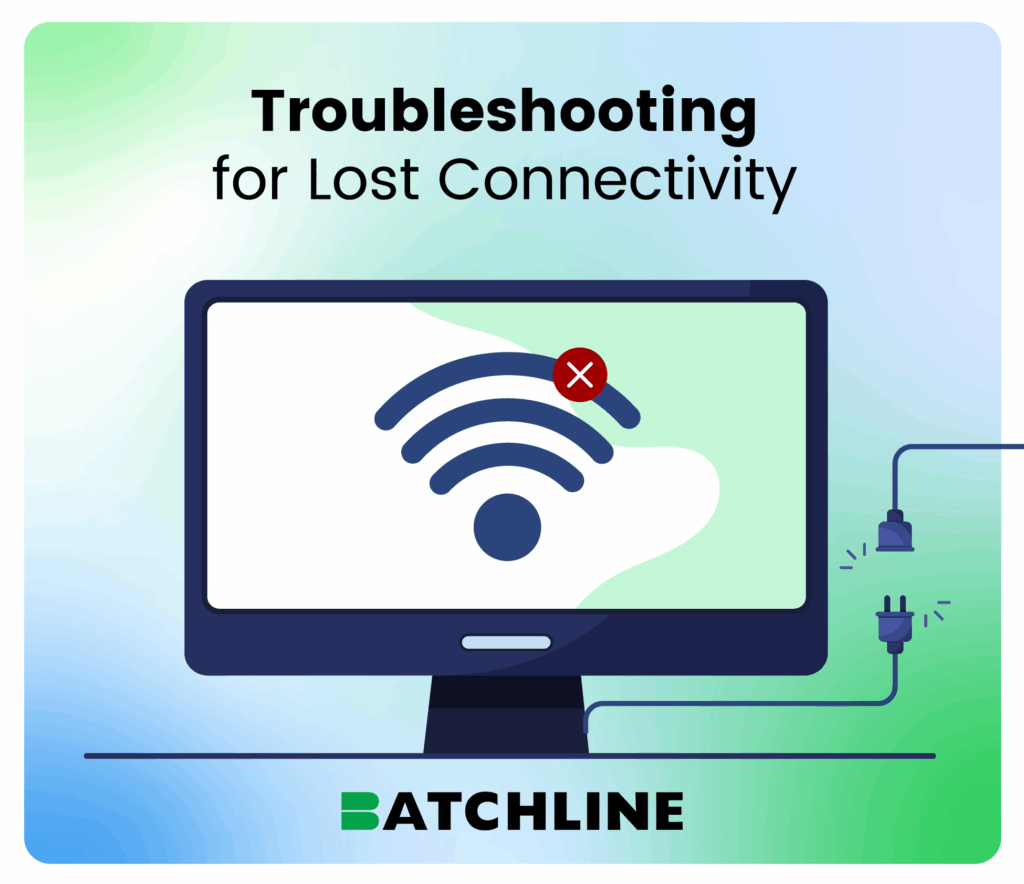
Handling Power and Connectivity Issues
In an outage, rest assured your data remains intact, thanks to BatchLine’s secure save functionality.
Should connectivity be compromised, operations can pivot to manual PDF specifications until normalcy is restored, integrating records post-fix.
Support and Implementation
Support Availability and Response Times
Our Thailand-based support team typically operates from 9 am to 6 pm (GMT+7), accommodating local business hours globally to ensure uninterrupted assistance.
Our 24/7 support option is available for an extra fee, with response times prioritized based on issue criticality.
Implementation Timeline and Onsite Assessment
Expect a timeline of 4-6 months for ERP-integrated EBR scopes, contingent on interface readiness. We offer onsite assessments to evaluate machine capabilities for seamless project integration, generally achievable in one day.

Training and Upgrades
Comprehensive User Training
Our training sessions, spreading across 3-5 days, empower operators, supervisors, and administrative roles with essential skills for batch execution, approval, and system management.
Customization and Upgrades
Custom system enhancements are available at additional costs. We release system upgrades biannually, encouraging users to stay current within Virtual Private Cloud (VPC) environments to leverage improved performance and features.

BatchLine Lite MES
Stands as a cost-effective choice for pharmaceutical manufacturers striving to optimize their processes and impeccable compliance.
Whether you manage a large-scale operation or a budding enterprise, contact us today to explore a personalized demo of BatchLine Lite MES.
Let our experts guide you through tailoring this eBMR solution to meet and exceed your organization's unique requirements.
Contact us today