BatchLine Lite MES
Smart eDHR solution for medical device manufacturing — paperless, traceable, and compliant with global standards like FDA 21 CFR 820.184, Part 11 & ISO 13485.
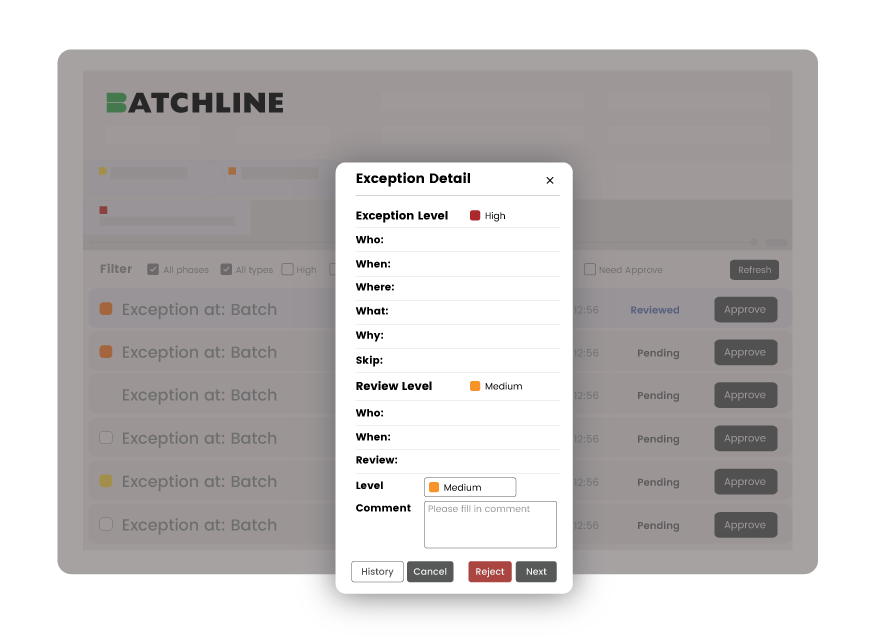
Review by exception
Slash batch review
&
Approval time in half
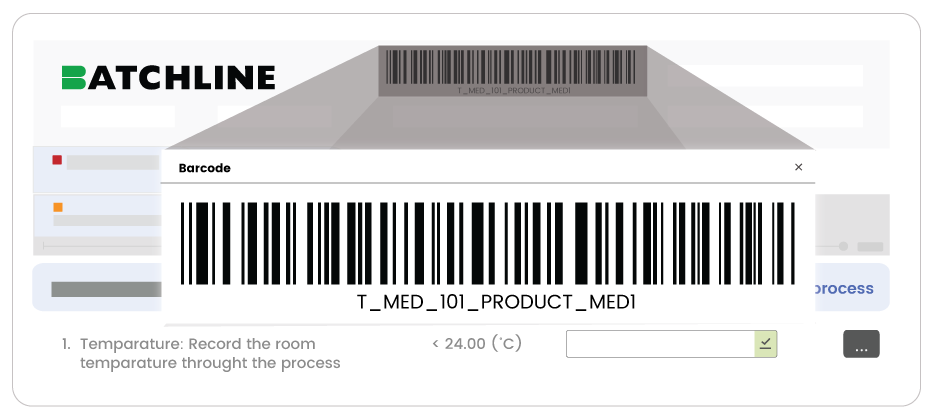
Barcode
This makes it simpler
to
identify & track
batches, helping
prevent mistakes
and saving time.
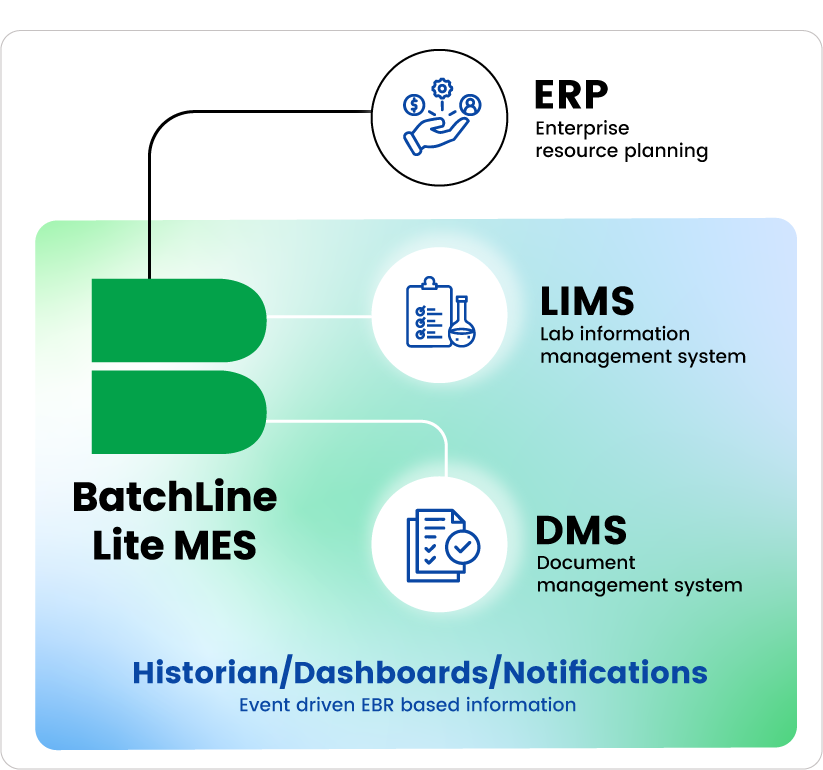
Integrations
Connect your systems —
ERP, Equipment &
Devices, QMS,
and more
— seamlessly.
Customer Story
Rapid Transformation
in Just 11 weeks!
30%
Reduction in product release time
20%
Enhanced efficiency in batch release & approval
"BatchLine has revolutionized our QA processes, giving us instant access to critical data and reducing manual errors. The 30% reduction in product release time gain allows us to deliver higher-quality products, faster."

Shannen Singh
Quality Assurance Department

"BatchLine impressed us with professionalism, responsiveness, and a simple product that fits our needs. Promised a 12-week implementation, we achieved Go Live in under 11 weeks. With one dedicated resource, supported by IT, quality, and production teams, we collaborated with BatchLine using their templated documentation, which required minimal changes."

Jason Hayes
Head of Quality




